Understanding Differential Scanning Calorimetry: A Comprehensive Guide
Differential Scanning Calorimetry (DSC) is a pivotal thermal analysis technique used in science and industry to measure the temperatures and heat flows associated with thermal transitions in a material. This innovative method provides invaluable insights into the physical and chemical properties of substances, such as polymers, pharmaceuticals, and foods. In this comprehensive guide, we’ll delve into the fundamentals of DSC, its applications, and why it’s a critical tool in material characterization.
What is Differential Scanning Calorimetry (DSC)?
Differential Scanning Calorimetry is an analytical technique that measures the difference in the amount of heat required to increase the temperature of a sample and reference as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. The reference is typically a material with no transition over the temperature range of interest. The principle behind DSC is straightforward: it monitors heat effects associated with phase transitions and reactions as a function of temperature.
How Does DSC Work?
The core of the DSC operation involves heating or cooling a sample and an inert reference at a controlled rate. As the temperature changes, the sample may undergo physical or chemical changes that involve a change in enthalpy (heat content). These changes can include melting, crystallization, glass transitions, and chemical reactions. The DSC instrument measures the difference in heat flow between the sample and the reference, which is then plotted against temperature to create a DSC curve. This curve provides critical information about the thermal transitions in the sample.
Applications of Differential Scanning Calorimetry
DSC is a versatile technique with a wide range of applications across various fields:
- Pharmaceuticals: Characterization of drug substances, including polymorphism, melting point determination, and stability studies.
- Polymers: Determination of crystallinity, glass transition temperatures, and material compatibility.
- Food Science: Analysis of fats and oils, protein denaturation, and gelatinization of starch.
- Environmental Science: Studying thermal decomposition and oxidative stability of materials.
Why is DSC Important?
DSC is indispensable in research and quality control because it provides vital information that cannot be obtained by other means. It helps in:
- Material Development: Optimizing formulations and processing conditions.
- Quality Control: Ensuring consistency and performance of materials.
- Regulatory Compliance: Providing necessary data for regulatory submissions.
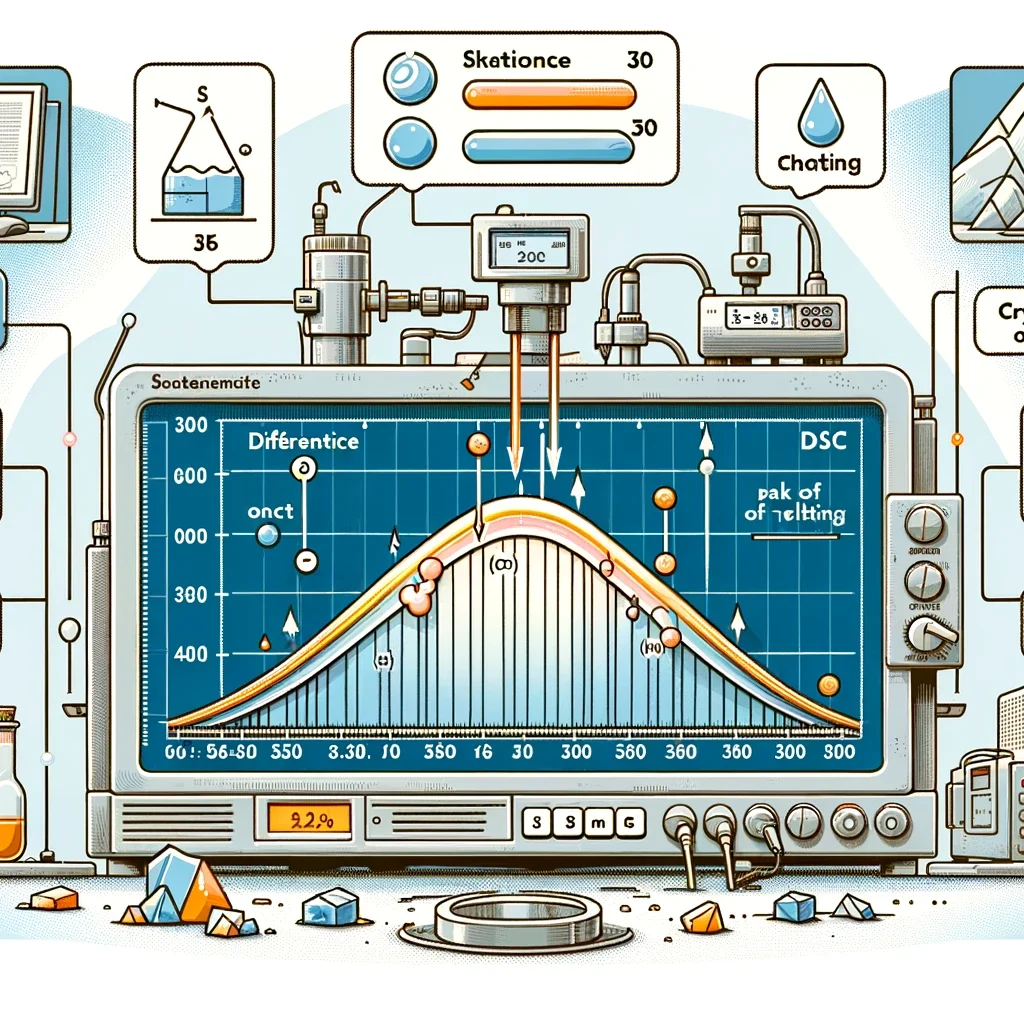
Visualizing DSC: An Illustrative Approach
To better understand the DSC process and its outcomes, let’s visualize a typical DSC curve. This curve represents a hypothetical material undergoing a melting transition followed by a crystallization on cooling.
- Onset of Melting: The point where the sample starts to melt.
- Peak of Melting: The temperature at which the melting is most significant.
- Crystallization: Observed upon cooling, indicating the formation of a crystalline structure from the liquid phase.
Conclusion
Differential Scanning Calorimetry is a cornerstone technique in the analysis of thermal properties of materials. Its ability to provide detailed insights into material behavior under thermal stress makes it an invaluable tool in both research and industry. Understanding DSC allows scientists and engineers to innovate and ensure quality in their materials, contributing significantly to advancements in various fields.
Incorporating DSC into your material analysis regime opens up a world of possibilities for characterization and development. Whether you’re in pharmaceuticals, polymers, food science, or environmental research, DSC offers the precision and reliability needed to push the boundaries of what’s possible with materials.
